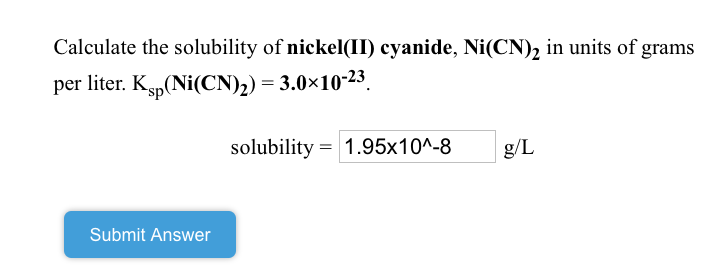![Photographs of the M( pz)[M'(CN) 4 ]·2H 2 O powders (M = Ni II , Co II... | Download Scientific Diagram Photographs of the M( pz)[M'(CN) 4 ]·2H 2 O powders (M = Ni II , Co II... | Download Scientific Diagram](https://www.researchgate.net/publication/283244109/figure/fig1/AS:355110174642179@1461676271493/Photographs-of-the-M-pzMCN-4-2H-2-O-powders-M-Ni-II-Co-II-and-M-Ni-II.png)
Photographs of the M( pz)[M'(CN) 4 ]·2H 2 O powders (M = Ni II , Co II... | Download Scientific Diagram
![Magnetic moment of [Ni(CN)4]2- is zero and that of [NiCl4]2- corresponds to 2 unpaired electrons Predict the geometry of - Chemistry - Coordination Compounds - 1176350 | Meritnation.com Magnetic moment of [Ni(CN)4]2- is zero and that of [NiCl4]2- corresponds to 2 unpaired electrons Predict the geometry of - Chemistry - Coordination Compounds - 1176350 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_5a26e80693bfb.png)
Magnetic moment of [Ni(CN)4]2- is zero and that of [NiCl4]2- corresponds to 2 unpaired electrons Predict the geometry of - Chemistry - Coordination Compounds - 1176350 | Meritnation.com
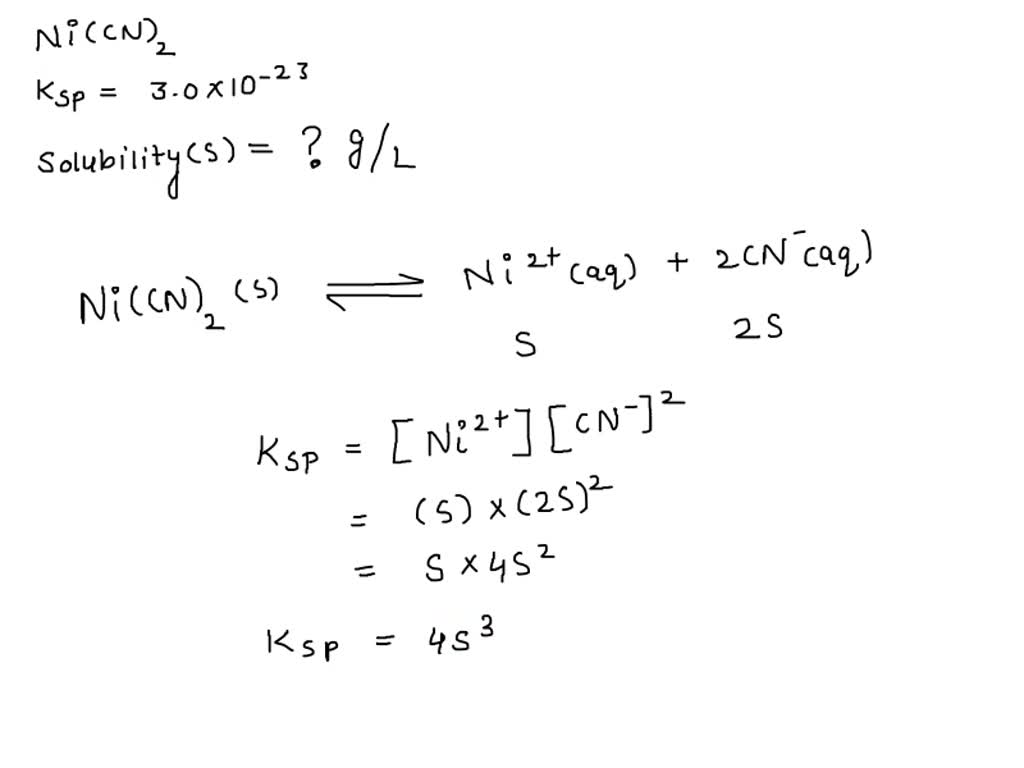
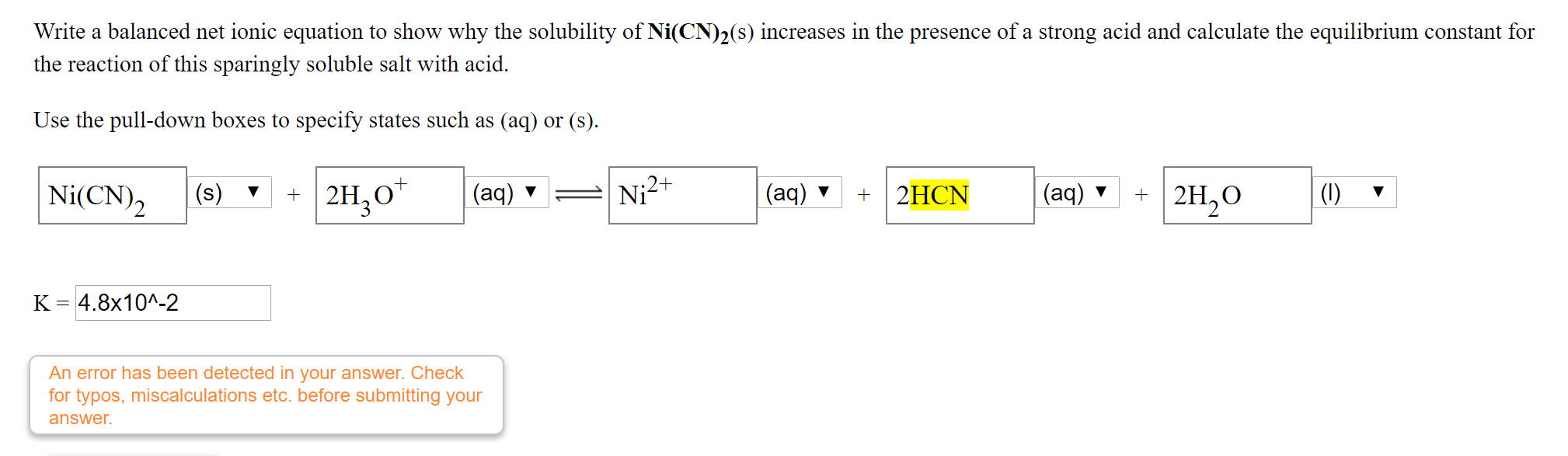
SOLVED: Calculate the solubility of nickel(II) cyanide, Ni(CN)2 in units of grams per liter: Ksp(Ni(CN)2) = 3.0x10^-23. solubility 1.95x10^-8 g/L Submit Answer
![SOLVED: [Ni(CN)4]2- is a square planar molecule. Draw out the molecular orbital diagram utilizing crystal field splitting for the Nickel ion. You must include appropriate labels for each of the orbitals. ***Also SOLVED: [Ni(CN)4]2- is a square planar molecule. Draw out the molecular orbital diagram utilizing crystal field splitting for the Nickel ion. You must include appropriate labels for each of the orbitals. ***Also](https://cdn.numerade.com/ask_previews/f9e7bf45-c75d-4f8a-95ca-f6c4a4692617_large.jpg)
SOLVED: [Ni(CN)4]2- is a square planar molecule. Draw out the molecular orbital diagram utilizing crystal field splitting for the Nickel ion. You must include appropriate labels for each of the orbitals. ***Also
Ni(CN)4]^2- is diamagnetic, while [Ni(CN)4]^2- is paramagnetic, explain using crystal field theory. - Sarthaks eConnect | Largest Online Education Community
![Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species. - Zigya Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species. - Zigya](https://www.zigya.com/application/uploads/images/chen12070397-a_5714962c868f8.png?t=1460966959418)
Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species. - Zigya

Catalytic hydrocyanation of α-ketoalkynes by Ni(CN)2/CO/KCN system in alkaline aqueous media: Identification of the active species - ScienceDirect
![Explain hybridisation, geometry and magnetic property of [Ni(CN)4]2− ion using Valence Bond Theory(VBT). [Atomic number of Ni is 28]. Explain hybridisation, geometry and magnetic property of [Ni(CN)4]2− ion using Valence Bond Theory(VBT). [Atomic number of Ni is 28].](https://search-static.byjusweb.com/question-images/toppr_ext/questions/874625_947137_ans_e945a0328151431c8601af56eaa7dcb3.png)
Explain hybridisation, geometry and magnetic property of [Ni(CN)4]2− ion using Valence Bond Theory(VBT). [Atomic number of Ni is 28].
![The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com](https://homework.study.com/cimages/multimages/16/cms53022462554618608855.jpg)
The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com

![What is the hybridization for [NiCN4]2 ? What is the hybridization for [NiCN4]2 ?](https://byjus-answer-creation.s3.amazonaws.com/uploads/2.14.jpg_img_upload_solution_2022-05-30%2005:07:29.453226.png)
![Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube](https://i.ytimg.com/vi/5S_my6-2Vkc/maxresdefault.jpg)
![The complex ion [Ni(CN)4]2− is : The complex ion [Ni(CN)4]2− is :](https://search-static.byjusweb.com/question-images/toppr_ext/questions/623286_597576_ans_83f06d1a64a9465c875f91d8efb8e27d.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)


![Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:ffa0132b7d904ae4be8cc8b622fc0250.png)
![What is the hybridisation for [Ni(CN) 4] 2-? - Quora What is the hybridisation for [Ni(CN) 4] 2-? - Quora](https://qph.cf2.quoracdn.net/main-qimg-20bb619759136ec1b4419262dd5a28a0.webp)

![Explain on the basis of valence bond theory that [Ni(CN)4]2&ndash Explain on the basis of valence bond theory that [Ni(CN)4]2&ndash](https://www.zigya.com/application/zrc/images/qvar/CHEN12070035-1.png)
![coordination compounds - Electronic configuration in [Ni(CN)4]2- - Chemistry Stack Exchange coordination compounds - Electronic configuration in [Ni(CN)4]2- - Chemistry Stack Exchange](https://i.stack.imgur.com/znHnV.png)