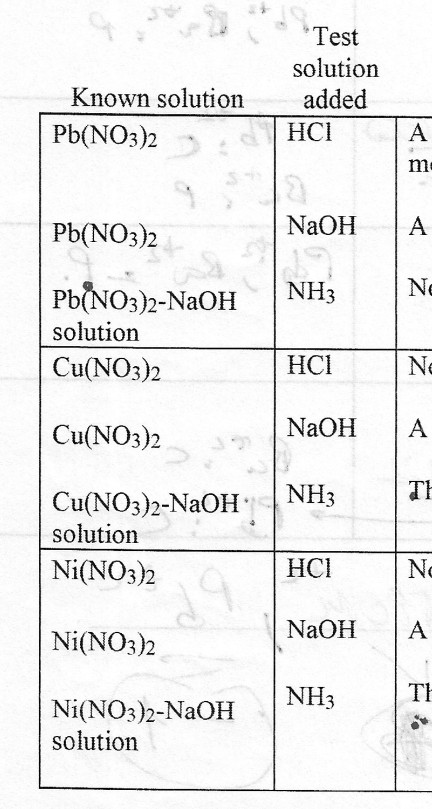
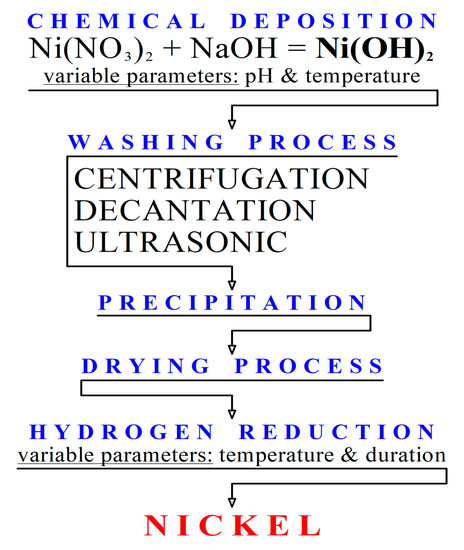
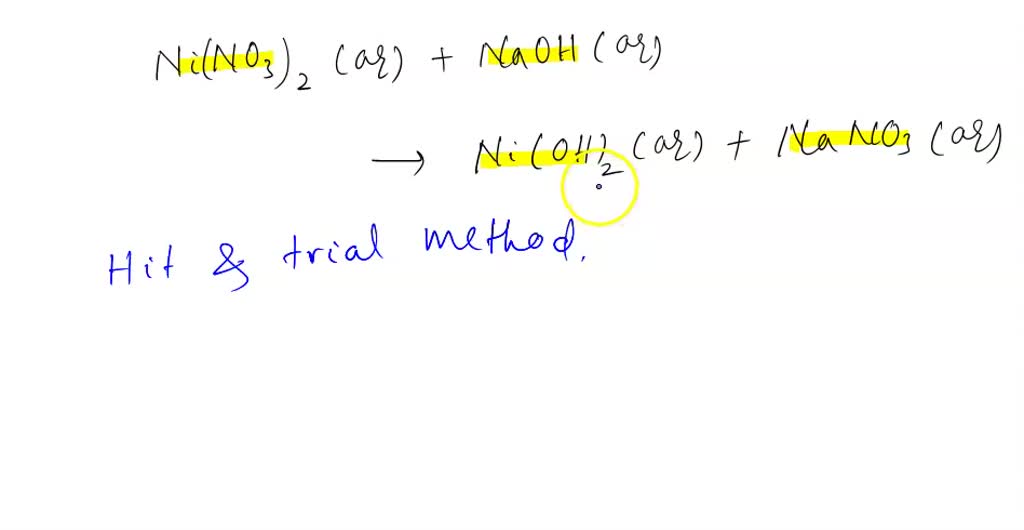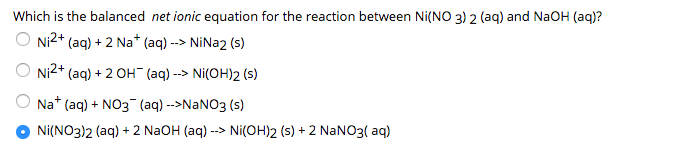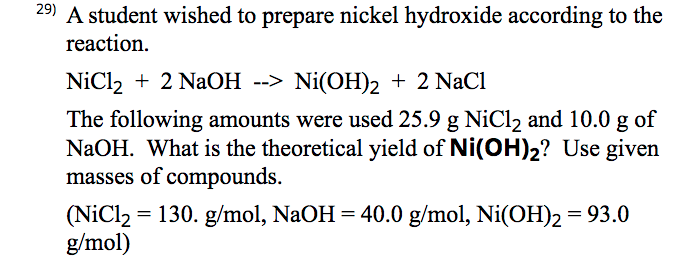Metals | Free Full-Text | Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media

Influence of NaOH, Ni/Al2O3 and Ni/SiO2 catalysts on hydrogen production from the subcritical water gasification of model food waste compounds - ScienceDirect

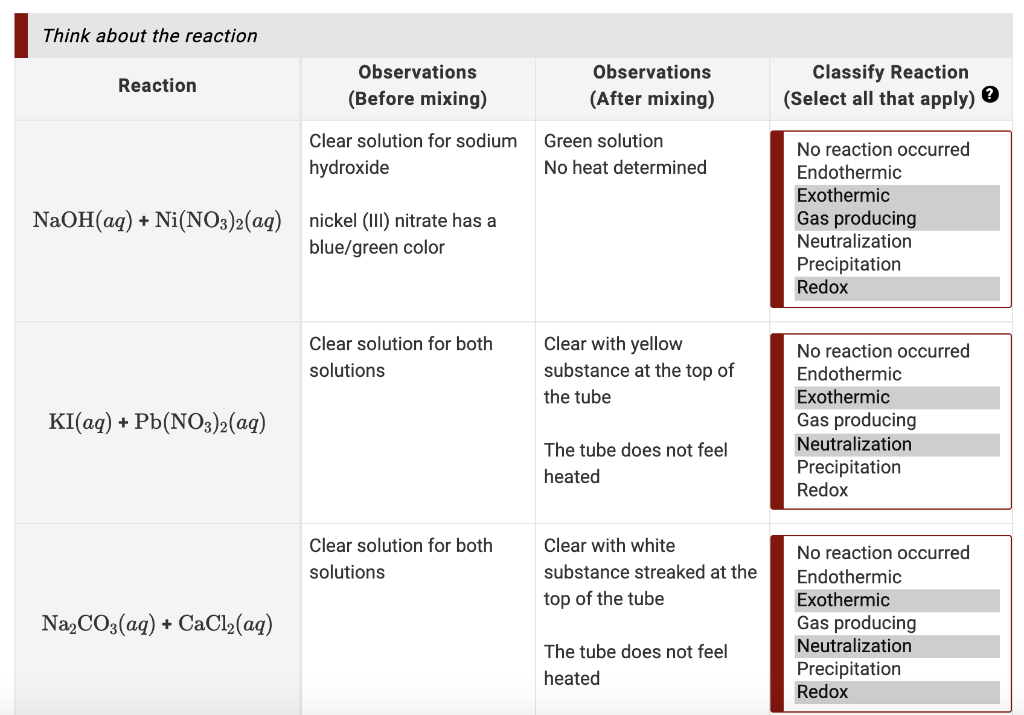
Chapter 4: Aqueous Reactions Solution: Solvent: substance present in the larger amount Solute: substance(s) dissolved in solvent, generally present in. - ppt download
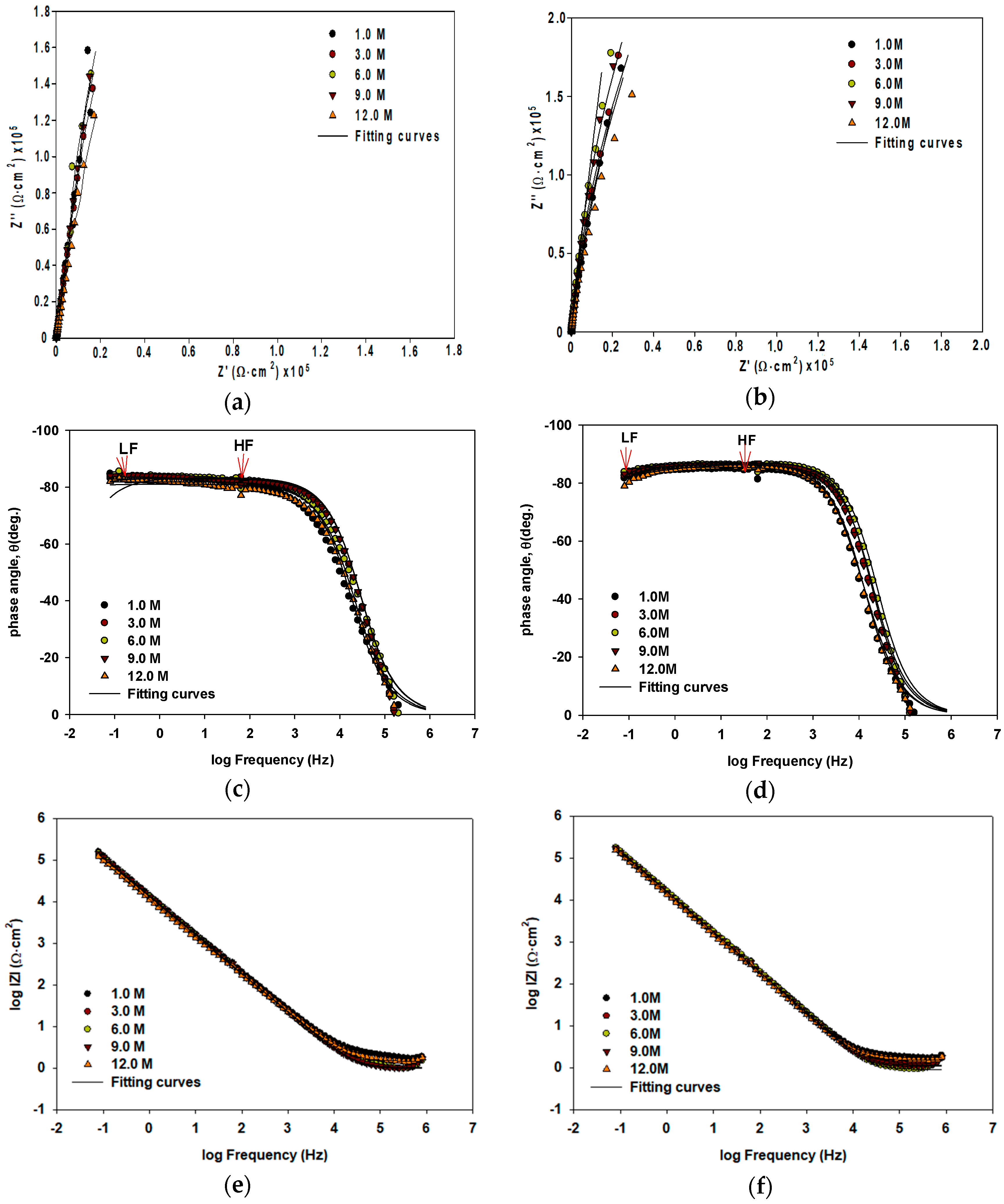
Metals | Free Full-Text | Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media

Strongly Coupled Ni/Ni(OH)2 Hybrid Nanocomposites as Highly Active Bifunctional Electrocatalysts for Overall Water Splitting | ACS Sustainable Chemistry & Engineering

Nickel hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing nickel ions. Nickel hydroxide (Ni(OH)2) is precipitated Stock Photo - Alamy

Engineering heterostructured Ni@Ni(OH)2 core-shell nanomaterials for synergistically enhanced water electrolysis - ScienceDirect

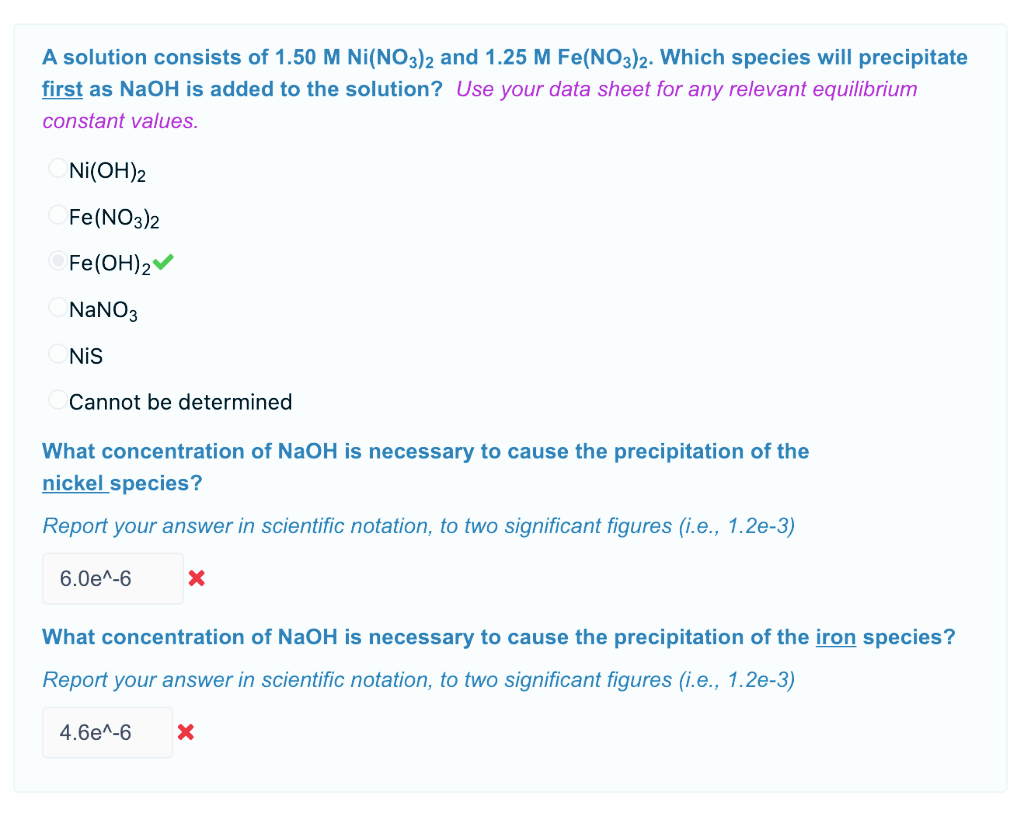
Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is..... - YouTube