![Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube](https://i.ytimg.com/vi/R5RDFu1oYUU/maxresdefault.jpg)
Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube
![Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number](https://i.pinimg.com/originals/cb/55/b9/cb55b933ee9ec4d59fdf9d226cf3a51a.png)
Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number
![C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B](https://toppr-doubts-media.s3.amazonaws.com/images/8783536/3c02ac26-f23b-4dda-96f6-23fb3b82533e.jpg)
C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B

Table 4 from Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate | Semantic Scholar

NH4BF4 is added to a 2 M NH3 solution and heated. This is then added to hexaamminenickel(II) ion solution. What is the product? | Homework.Study.com
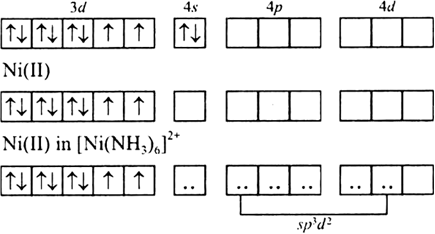
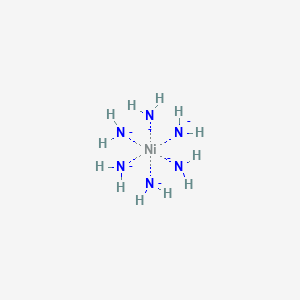
Coordination compound: Explain [Co(NH3)6]3+ is an inner orbital complex while [Ni(NH3)6]2+ is an outer orbital complex .

Inorganics | Free Full-Text | Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate
![SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light. SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.](https://cdn.numerade.com/ask_images/6feedc9774204ebeabd886be4a6d6f35.jpg)
SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.
![Explain the hybridisation, magnetic property and geometry [Ni(CN)4]2− and [ Ni(NH3)4]2+ using VB theory. Explain the hybridisation, magnetic property and geometry [Ni(CN)4]2− and [ Ni(NH3)4]2+ using VB theory.](https://search-static.byjusweb.com/question-images/toppr_ext/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
![Answered: [Ni (NH3) 6] C12 Explain the… | bartleby Answered: [Ni (NH3) 6] C12 Explain the… | bartleby](https://content.bartleby.com/qna-images/question/33dc3186-d7b9-4388-8560-215f799e8fe0/013f6e76-4df4-4063-bb5c-5eb17a580f72/z92qakc_thumbnail.png)
![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)


![Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby](https://content.bartleby.com/qna-images/answer/c73807b6-45ed-4420-a3b1-cee7bec70749/203ec893-0265-480e-b058-95e1378e904d/3cda3a30-aca8-11eb-99b8-c16e6153b0d2_IMG_20210504_124206_523~2.jpg)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-5256b41f5879418f6611bf7cb42000b7.webp)
2. The... | Download Scientific Diagram Representation of the cubic structure of [Ni(NH3)6](NO3)2. The... | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig8/AS:667921089036294@1536256205990/Representation-of-the-cubic-structure-of-NiNH36NO32-The-constituent-atoms.jpg)
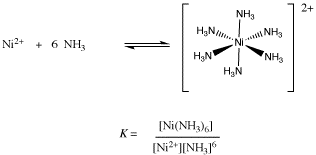
![Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)
![why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE](https://preview.redd.it/why-cant-ni-nh3-6-2-have-dsp3-hybridization-then-v0-slwfvbv55ria1.png?width=640&crop=smart&auto=webp&s=f4e658df50fc25da185b4ccd9590bfb69fc4c902)