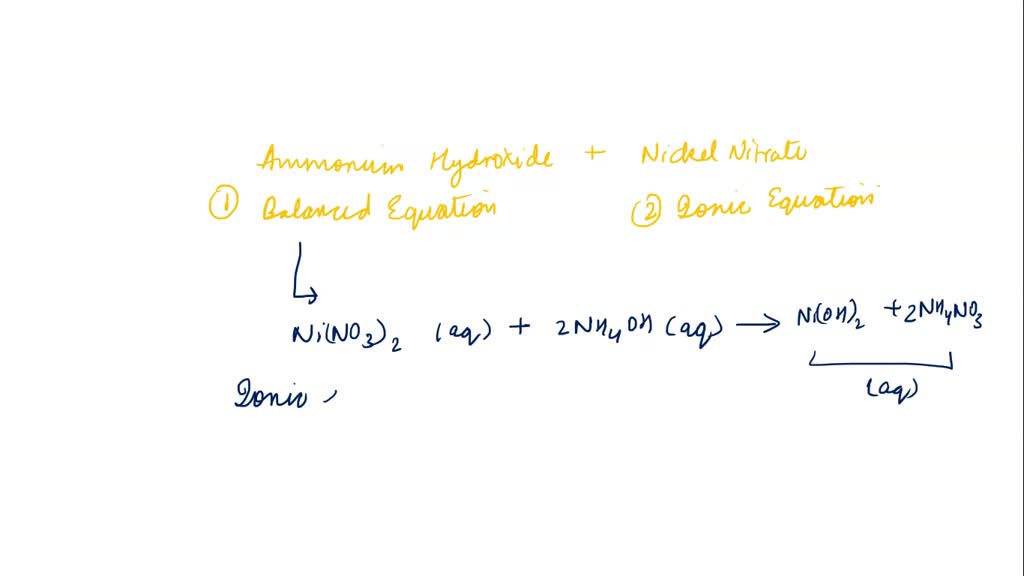
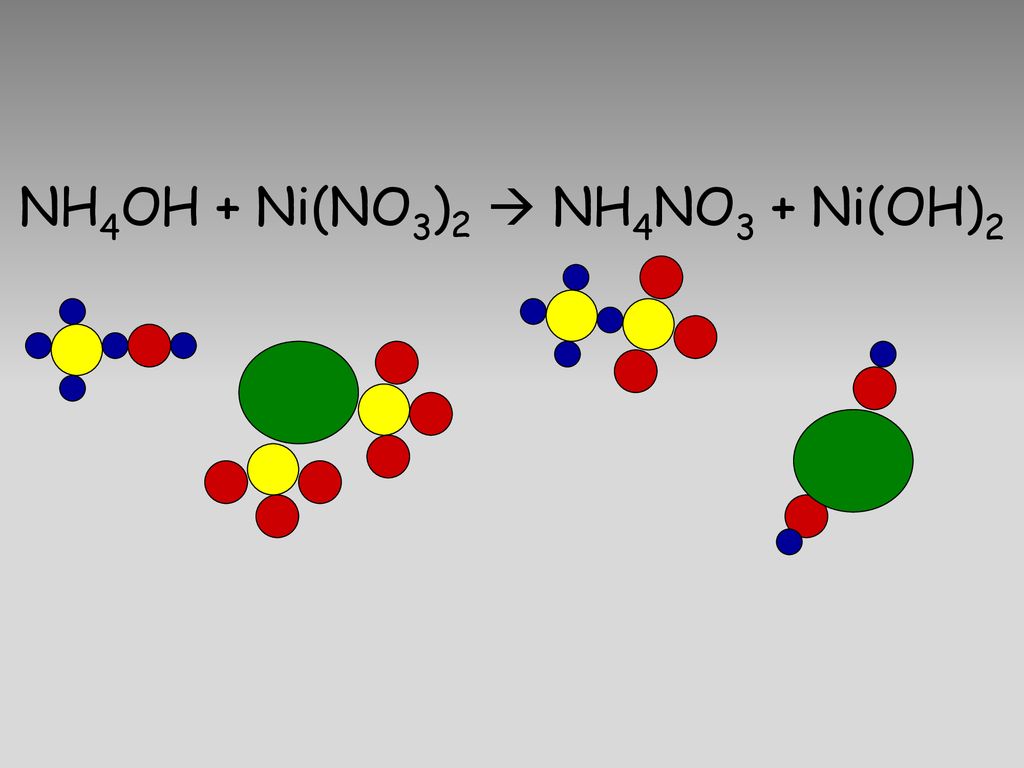
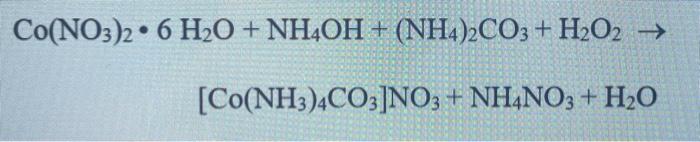A 1 litre solution containing NH4Cl and NH4OH has hydroxide ion concentration of 10^-6 mol/litre. Which of the following hydroxides could be precipitated when the solution is added to 1 litre solution

TGA/DTA thermograms of Ni(OH) 2 prepared (a) without and (b) with CTAB. | Download Scientific Diagram

Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect

E740: Equilibrium – Complex Ions – Metal + Ammonia Complexes | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect
27. DMG +NiCl2+NH4OH makes Complex a+ NH4Cl+H2O. What is complex a and find the hybridisation magnetic character and Oxidation state of Nickel in complex a ?
29. NiCl2 + NH4OH + dimethylglyoxime ——> A ( complex ) Incorrect statement for complex A is /are 1 Coordination number of metal ion is 4 2 Two five membered and two

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Direct preparation of Al-substituted α-Ni(OH)2 from Al-containing salt solution by immersing method - ScienceDirect